Sevodyne meets all the relevant regulatory requirements of a bioequivalent medicine (reference product: Norspan® [(buprenorphine patches) Mundipharma Gmbh, Germany]/BuTrans® [(buprenorphine patches) Napp Pharmaceuticals Ltd, UK]).1 Two bioequivalence studies were performed, one single-dose study and a multi-dose study, and an adhesion study was also performed.1
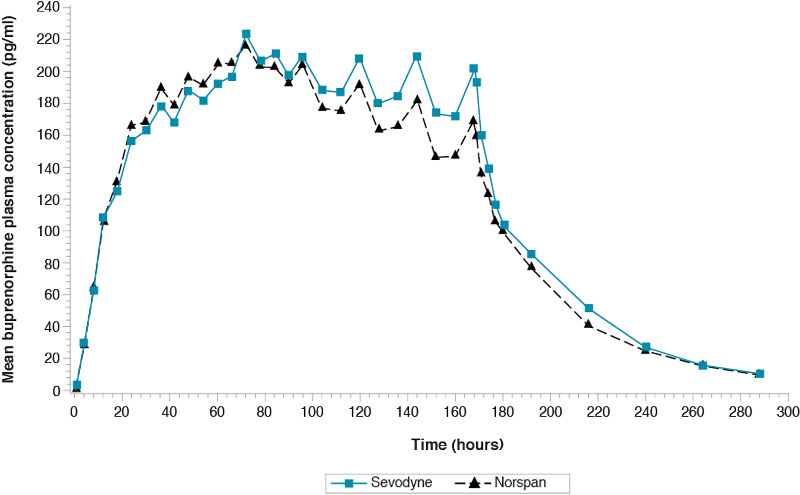
Single-dose study comparing Sevodyne 20 microgram/hr transdermal patch and Norspan 20 microgram/hr transdermal patch
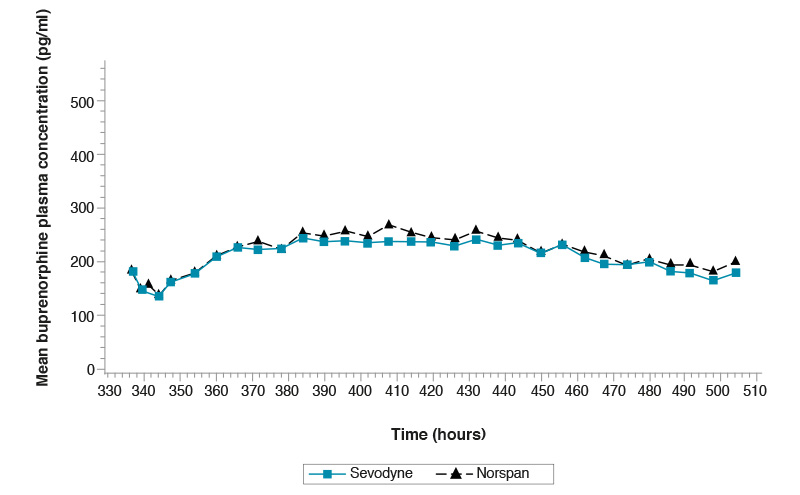
Multiple-dose study comparing Sevodyne 20 microgram/hr transdermal patch
and Norspan 20 microgram/hr transdermal patch
The adhesion study showed that both Sevodyne and Norspan had overall mean adherence (over 168 hours of patch wear) higher than 90%.1
References: 1) Data on file, 1010067149 v 5.0 September 2021
SEV1010311E1_NOV2022













